Immunogenicity and reactogenicity of co-administered tetanus–diphtheria–acellular pertussis (Tdap) and tetravalent meningococcal conjugate (MCV4) vaccines compared to their separate administration - ScienceDirect
Par un écrivain mystérieux
Description

PDF) Immunogenicity and safety of the quadrivalent meningococcal vaccine MenACWY-TT co-administered with a combined diphtheria-tetanus-acellular pertussis vaccine versus their separate administration in adolescents and young adults: A phase III

Collection, compilation and analysis of bacterial vaccines - ScienceDirect

Collection, compilation and analysis of bacterial vaccines - ScienceDirect

Vaccines - ScienceDirect
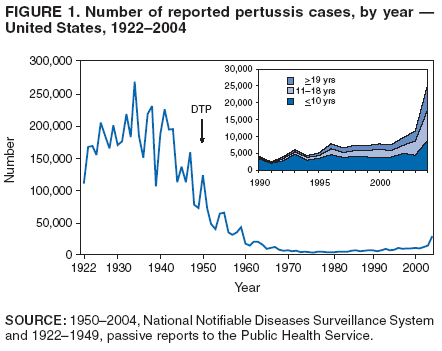
Preventing Tetanus, Diphtheria, and Pertussis Among Adolescents: Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccines
Recommendations of the Advisory Committee on Immunization Practices (ACIP)

PDF) Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (MenACYW-TT) in adolescents and adults: A Phase III randomized study

The adjuvanted recombinant zoster vaccine co-administered with a tetanus, diphtheria and pertussis vaccine in adults aged ≥50 years: A randomized trial - ScienceDirect
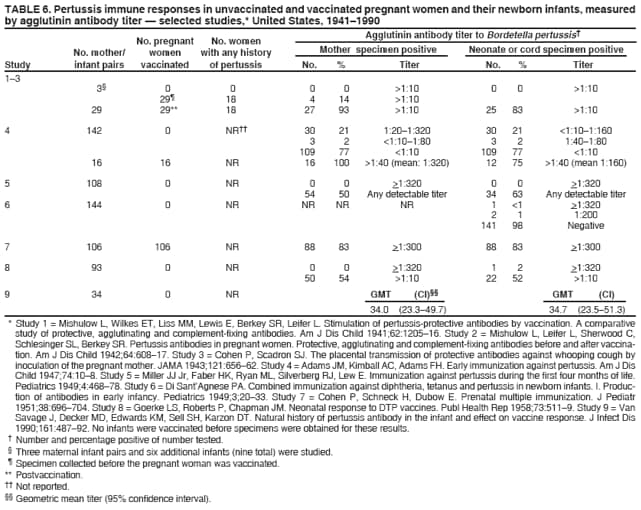
Prevention of Pertussis, Tetanus, and Diphtheria Among Pregnant and Postpartum Women and their Infants Recommendations of the Advisory Committee on Immunization Practices (ACIP)

Immunogenicity and reactogenicity of Infanrix™ when co-administered with meningococcal MenACWY-TT conjugate vaccine in toddlers primed with MenHibrix™ and Pediarix™ - ScienceDirect

Effect of Tdap when administered before, with or after the 13-valent pneumococcal conjugate vaccine (coadministered with the quadrivalent meningococcal conjugate vaccine) in adults: A randomised controlled trial - ScienceDirect
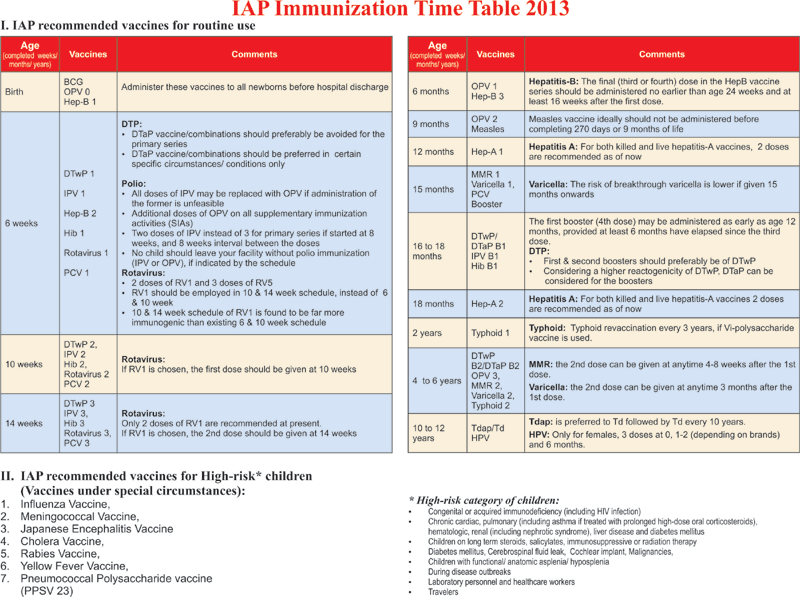
Indian Academy of Pediatrics (IAP) Recommended Immunization Schedule for Children Aged 0 through 18 years – India, 2013 and Updates on Immunization

Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines - ScienceDirect

PDF) Immunogenicity and safety of a quadrivalent meningococcal polysaccharide CRM conjugate vaccine in infants and toddlers
depuis
par adulte (le prix varie selon la taille du groupe)