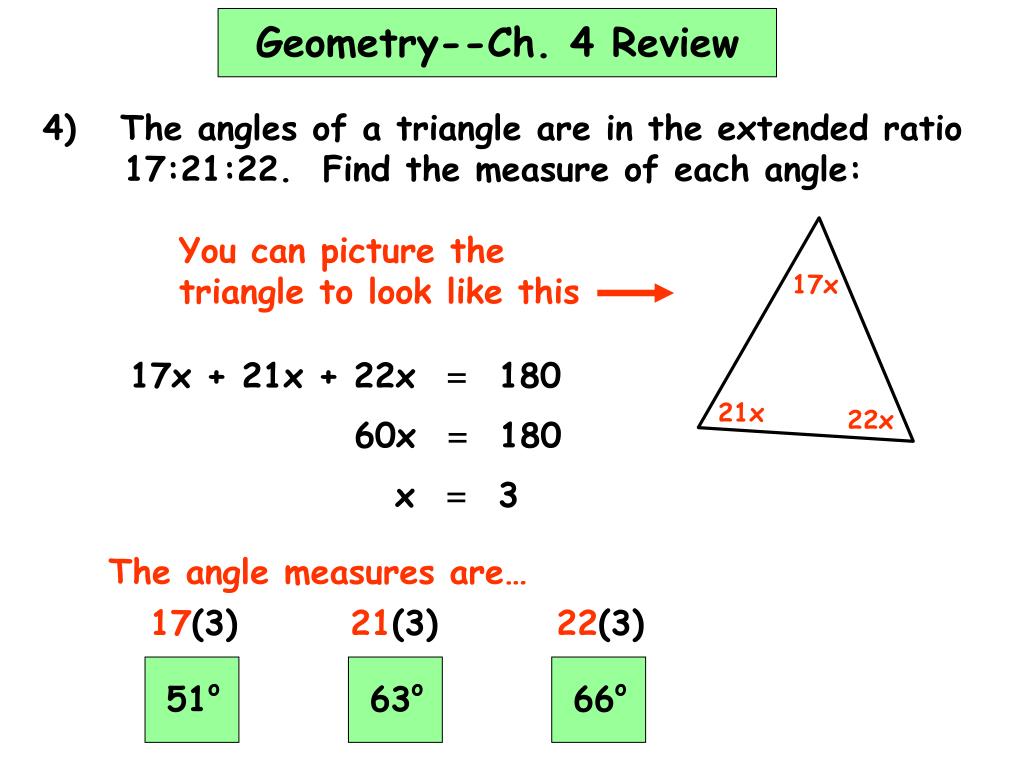
c) 20,000 200000 13. An element, X, have three isotopes 22X. The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be ((a) 9% (b) 8% (
Par un écrivain mystérieux
Description
Click here:point_up_2:to get an answer to your question :writing_hand:c 20000let 20000013 an element x have three isotopes22x the percentage abundance ofits average atomic
Click here👆to get an answer to your question ✍️ -c- 20-000 200000 13- An element- X- have three isotopes 22X- The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be -a- 9- -b- 8- -c- 10- -d- 0- have three isotopes 20X- 21X and age abundance of 20X is 90- and c mass of the element is 20-11-The
Click here👆to get an answer to your question ✍️ -c- 20-000 200000 13- An element- X- have three isotopes 22X- The percentage abundance of its average atomic mass of the element percentage abundance of 21X should be -a- 9- -b- 8- -c- 10- -d- 0- have three isotopes 20X- 21X and age abundance of 20X is 90- and c mass of the element is 20-11-The
Neon has two major isotopes, -20 and neon -22. What's the atomic

12345-Elements of Physical Metallurgy, PDF, Heat Treating

Q An element x has three isotopes x20,x21,x22. The percentage

An element X has three isotopes X^20, X^21 and X^22. The
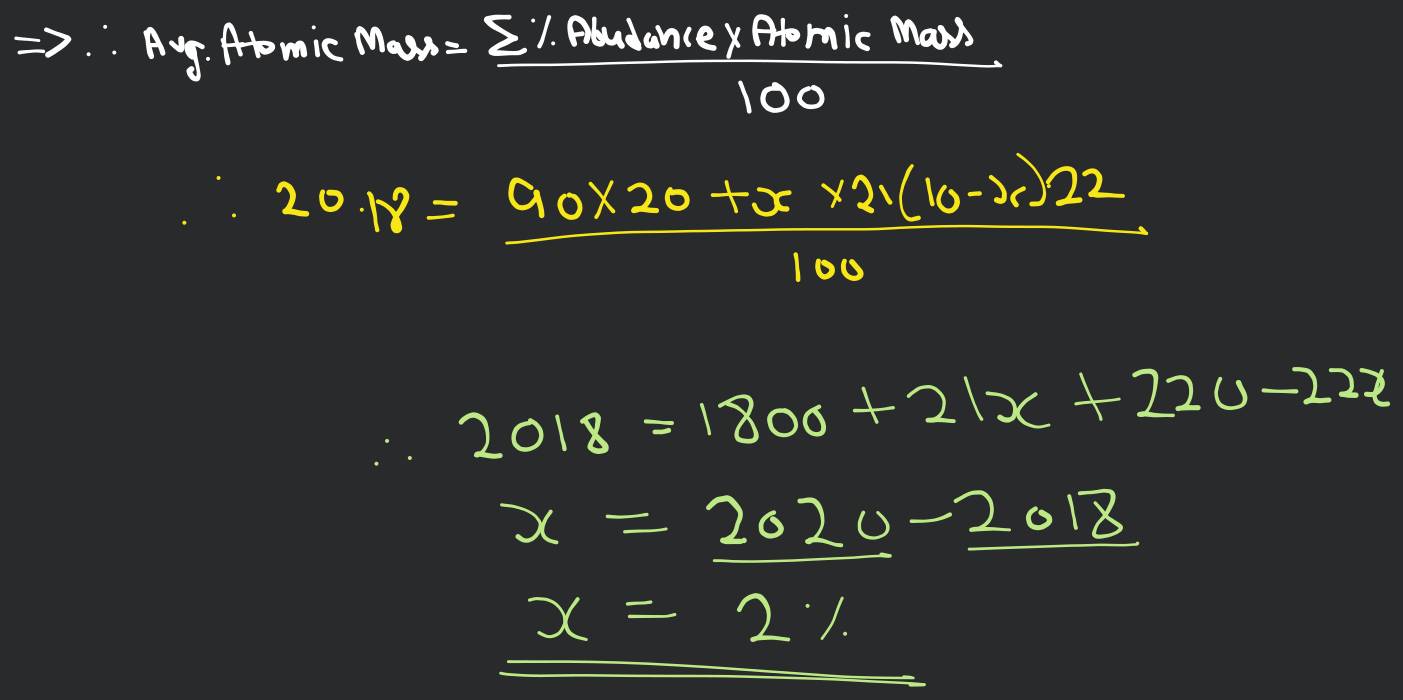
Q An element x has three isotopes x20,x21,x22. The percentage
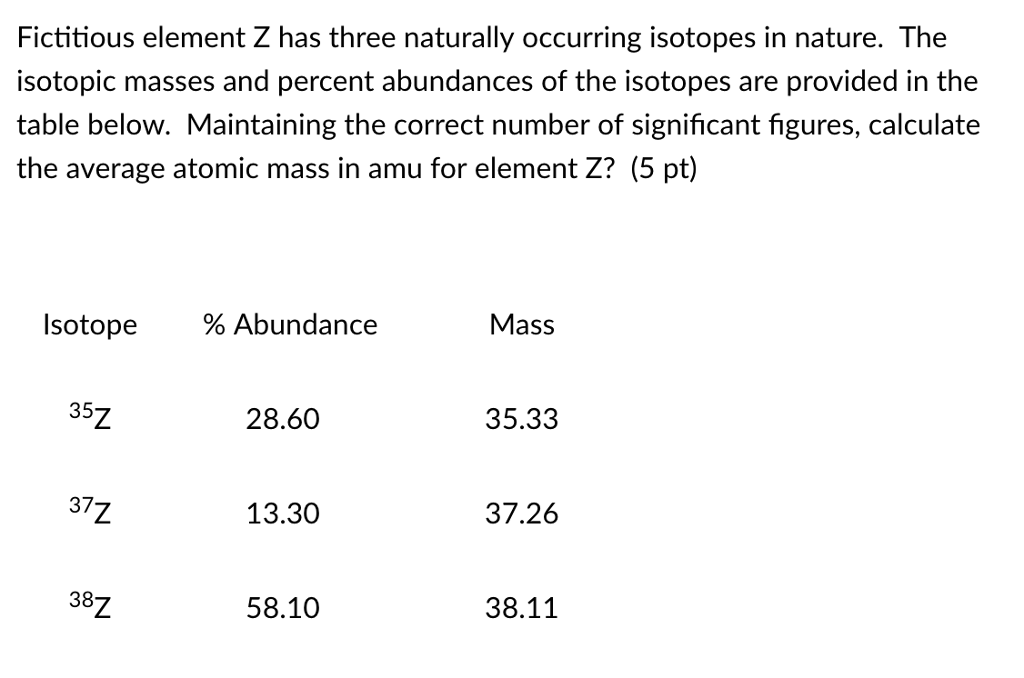
Solved Fictitious element Z has three naturally occurring
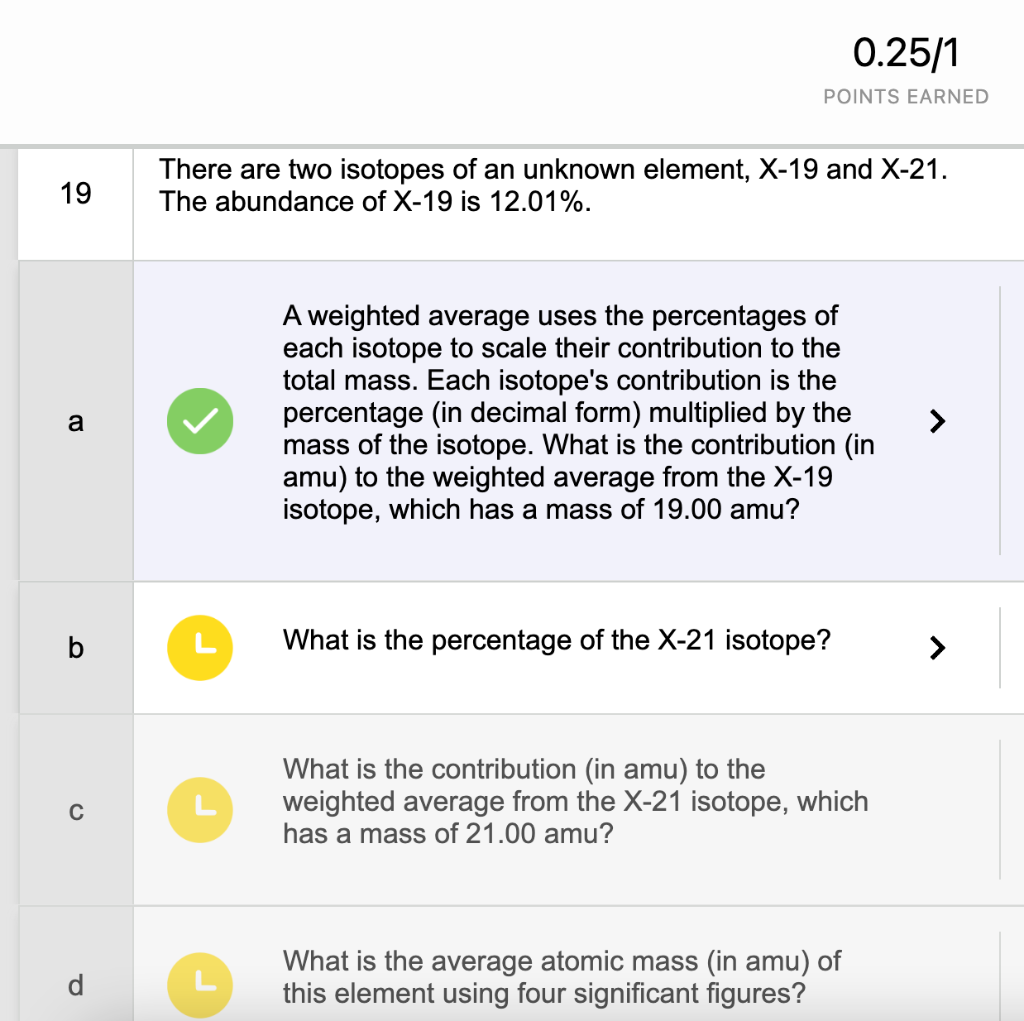
Solved There are two isotopes of an unknown element, X−19

61. An element X has two isotopes 41X and 43X. If percentage

Solved Element Z has three isotopes, which are listed in the

SOLVED: The element X has three naturally occurring isotopes. The

Finding Percent Abundance (3 Isotopes)

An element X has two major isotopes 69X and 71X which occur
depuis
par adulte (le prix varie selon la taille du groupe)