5.85g of NaCl is dissolved in 1L of pure water. The number of ions
Par un écrivain mystérieux
Description
5.85g of NaCl is dissolved in 1L of pure water. The number of ions in 1ml of this solution is

Chapter-2 Solution (DPP), PDF, Solution
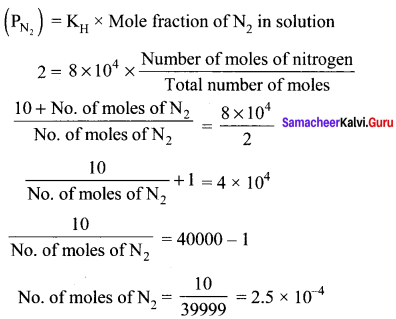
Samacheer Kalvi 11th Chemistry Solutions Chapter 9 Solutions – Samacheer Kalvi

Solutions For Physics and Chemistry, PDF, Osmosis
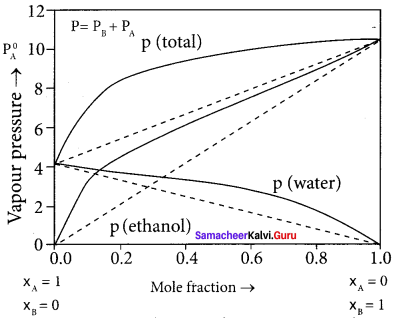
Samacheer Kalvi 11th Chemistry Solutions Chapter 9 Solutions – Samacheer Kalvi

How would one prepare one liter of 4 M NaCl solution?

Solutions Notes – Chemistry Classes / Ronald Reagan S.H.S.

PPT - Solutions PowerPoint Presentation, free download - ID:528392
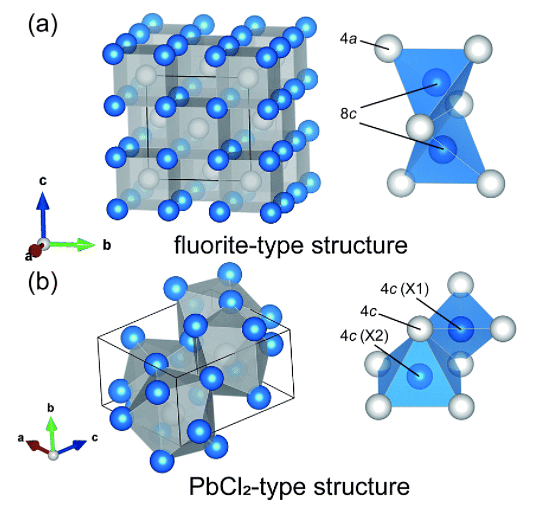
Lead II Chloride Formula: Properties, Structure, Examples

Module 2 - Solutions Flashcards

The lattice energy of NaCl is -786 kJ/mol, and the enthalpy of hydration of 1 mole of gaseous Na^+ and 1 mole of gaseous Cl^- ions is -783 kJ/mol. Calculate the enthalpy
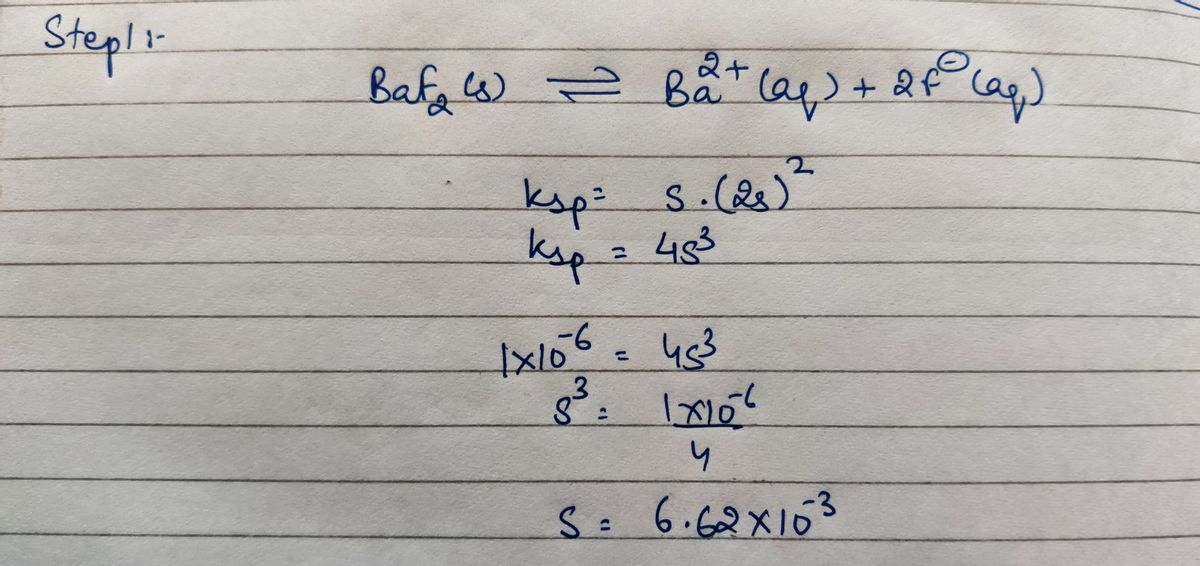
Answered: How many grams of BaF2 (molar mass =…

Molarity - Chemistry
depuis
par adulte (le prix varie selon la taille du groupe)