Heat of reaction for, CO(g) + 1/2 O2(g)→ CO2(g)at constant V is
Par un écrivain mystérieux
Description
29. For the reaction, 2CO + O2 = 2 CO : delta H = 560 kJ. Two moles of CO and one mole of O2 are taken in a container of volume

1st PUC Chemistry Question Bank Chapter 6 Thermodynamics - KSEEB Solutions

08 Chapter 13 (Compiled) PDF, PDF, Chemical Equilibrium
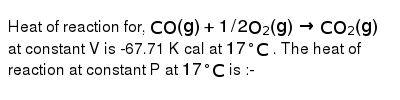
Heat of reaction for, CO(g)+1//2O(2)(g)rarr CO(2)(g) at constant V is
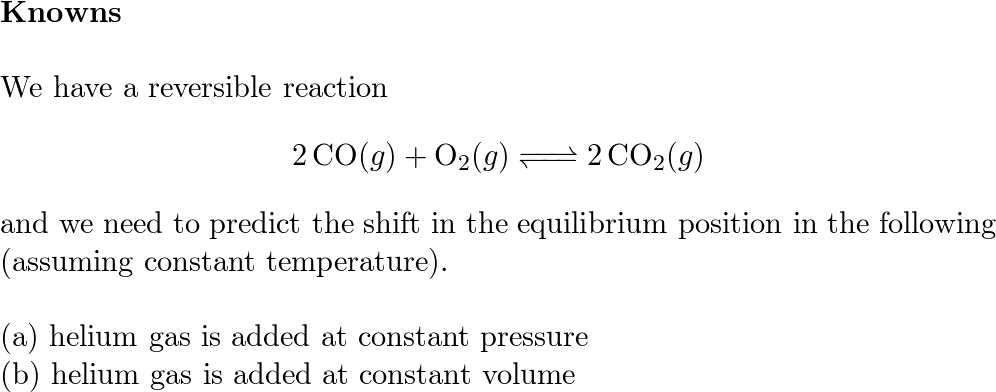
Consider the gas-phase reaction 2 CO (g) + O2 (g) <=> 2 CO2
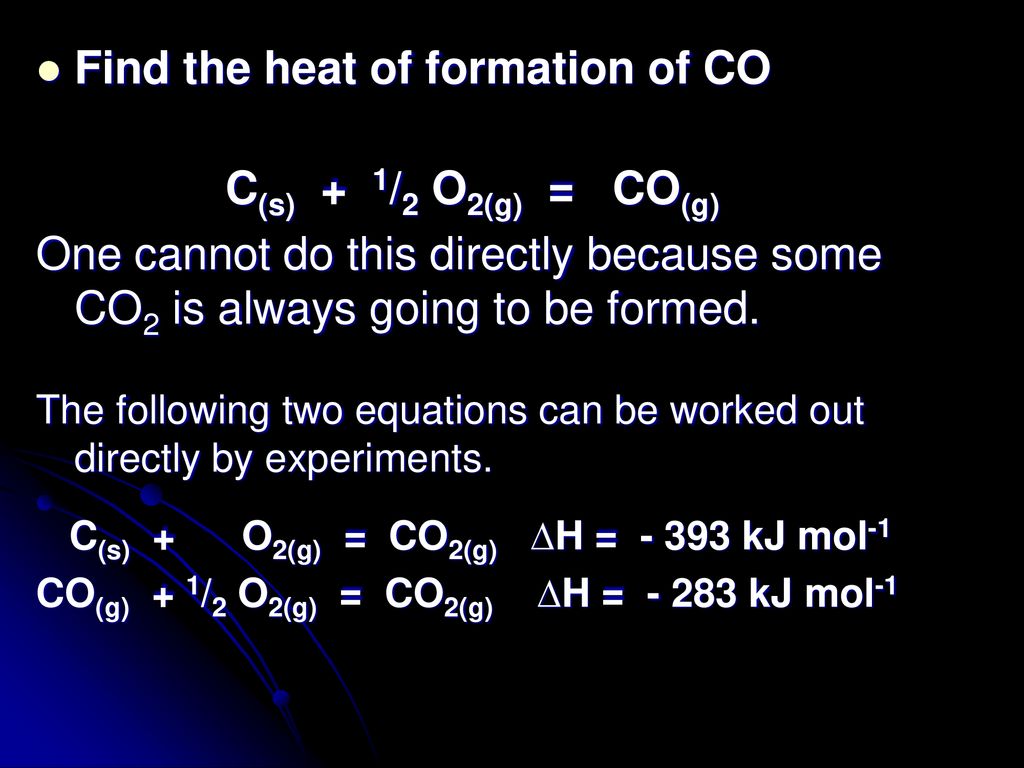
5.4.6 Hess's Law of Heat Summation [1840] - ppt download
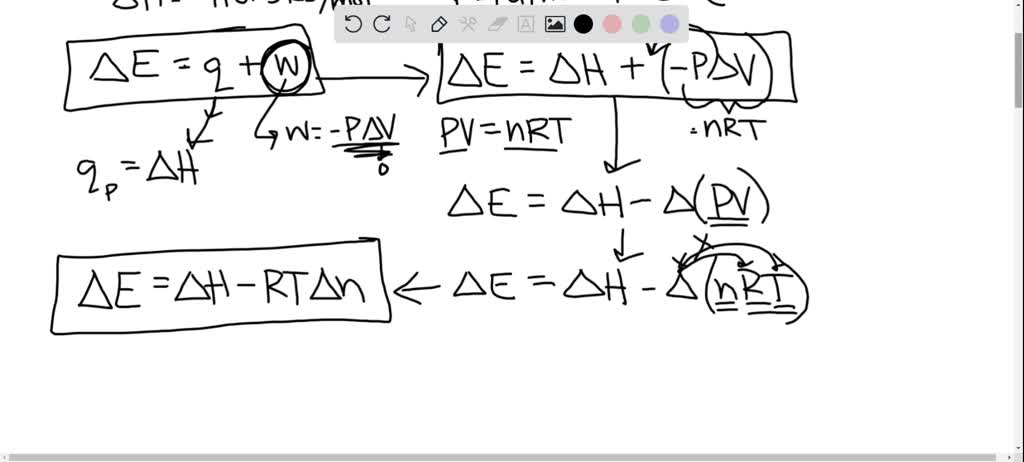
SOLVED: What is ΔE for the formation of one mole of CO at 1 atm and 25°C? C (graphite) + 1/2 O2(g) â†' CO(g) ΔH = -110.5 kJ/mol Please explain step-by-step.

Carbon monoxide - Wikipedia

Calculate the standard enthalpy of formation of CH3OH(l) from the following data :

Heat of reaction for; CO(g) + 1/2O2(g)→CO2(g) at constant V is - 67.71 cal 17^oC . The heat of reaction at constant P at 17^oC
Show that the reaction, CO(g) + 1/2 O2 (g) ----> CO2(g) at 300 K, is spontaneous and exothermic, - Sarthaks eConnect

5.7: Enthalpy Calculations - Chemistry LibreTexts
depuis
par adulte (le prix varie selon la taille du groupe)