Avoid Launch Delays By Planning For An FDA-Required REMS Risk
Par un écrivain mystérieux
Description
lt;p>Picture this: The FDA accepts a manufacturer's NDA, and the manufacturer plans for its impending launch. But shortly before the anticipated approval, the FDA notifies the manufacturer that a Risk Evaluation and Mitigation Strategy (REMS) program is required to market the product. Now what?</p>

2 Incorporating Benefit and Risk Assessment and BenefitRisk Management into Food and Drug Administration Decision-Making, Ethical and Scientific Issues in Studying the Safety of Approved Drugs

White Paper, Missed Opportunities When Developing a REMS Program
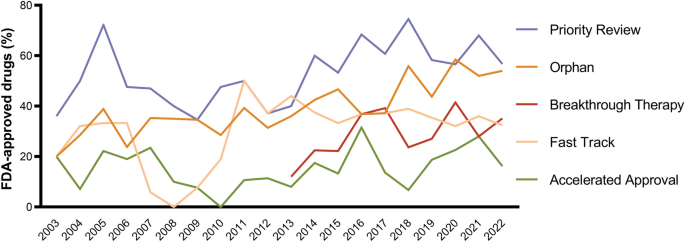
Special FDA designations for drug development: orphan, fast track, accelerated approval, priority review, and breakthrough therapy
PDF) Risk Evaluation and Mitigation Strategies (REMSs): Are They Improving Drug Safety? A Critical Review of REMSs Requiring Elements to Assure Safe Use (ETASU)

Improving Risk Evaluation and Mitigation Strategy - Cognizant
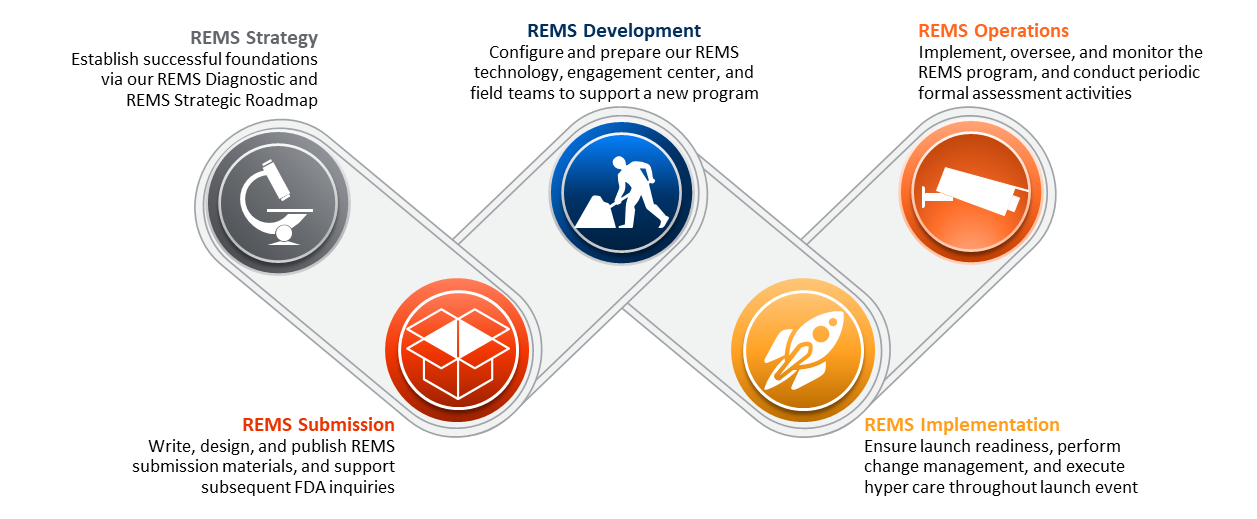
Full-Service Risk Evaluation & Mitigation Strategy (REMS) Solutions

Roadmap to risk evaluation and mitigation strategies (REMS) success - John D. Balian, Janice C. Wherry, Rachpal Malhotra, Valerie Perentesis, 2010

REMS Modernization Can't Wait A Call to Action
From Our Perspective, A Two-Part Series: Risk Evaluation and Mitigation Strategies (REMS) Program

Psychedelic drug abuse potential assessment research for new drug applications and Controlled Substances Act scheduling - ScienceDirect

Chimeric Antigen Receptor T-Cell Therapies: Barriers and Solutions to Access

FDA Struggles with Risk Management and Drug Safety
depuis
par adulte (le prix varie selon la taille du groupe)







